Since the global epidemic era has entered, various industries have also been affected to varying degrees. Driven by the Omicron variant, the global outbreak continues, resulting in a daily increase in the number of infected people in various countries. According to WHO statistics, the number of newly diagnosed patients has increased to 476,374,234 worldwide as of March 25, 2022.
In order to end the spread of Coronavirus pandemic as soon as possible and return people's lives to normal, it is also means that a large part of the world's population needs to be immunized against this virus. To achieve this goal, the safest method is to use vaccines. (Vaccines are a technology that humans have often relied on in the past to reduce the number of deaths from infectious diseases.)
According to Our World in Data, 64.2% of the world's population has received at least one dose of COVID-19 vaccine. Globally, 11.14 billion doses have been administered, and 16.14 million doses are now administered daily. Only 14.4% of people in low-income countries have received at least one dose of the vaccine.
Vaccination against covid-19 is the most important step in preventing infection, but masking and testing for covid-19 are also important tools to slow the spread of covid-19.
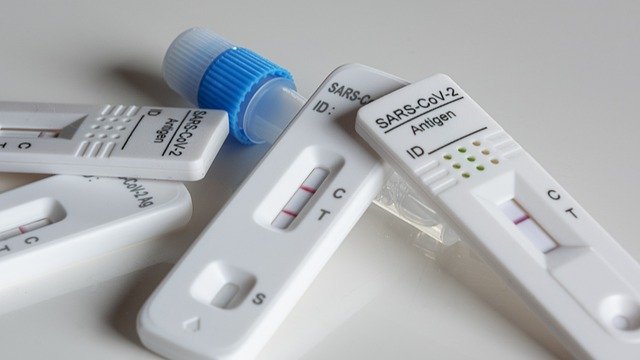
The outbreak of the Coronavirus has also led to the emergence of Coronavirus testing products. There are currently three main methods of testing for Coronavirus in the world, which are nucleic acid testing, antibody testing, and antigen testing.
Nucleic acid testing and antigen testing are commonly used to detect viruses, with antigen testing being faster than nucleic acid testing, but far less accurate.
Nucleic acid testing is more recognized worldwide as the gold standard for coronavirus-19 detection. Antibody testing, on the other hand, is usually an adjunct to coronavirus testing.
Commonly available products for coronavirus testing include antigen and antibody kits, nucleic acid testing instruments and related consumables, and there is a growing demand for these products in various countries.
Currently, certified coronavirus testing products include Cue COVID-19 Test (Cue Health Inc), Abbott RealTime SARS-CoV-2 RT-PCR Kit (Abbott Molecular), GenePro SARS-CoV-2 Tes (Gencurix, Inc), etc.
And there is another covid rapid test product-
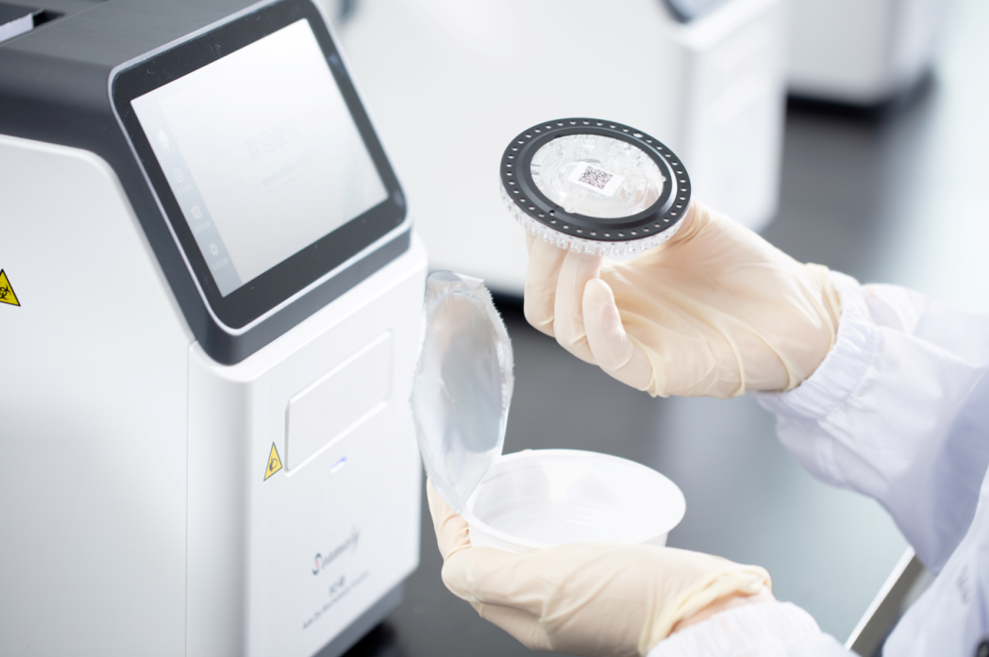
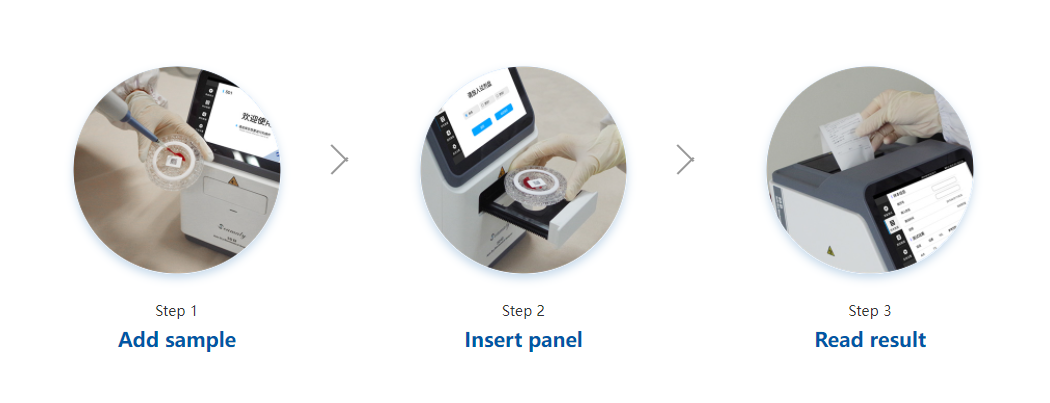
Pluslife Mini Dock (Seamaty). Pluslife Mini Dock is a COVID-19 rapid test instrument developed and manufactured by Pluslife.
It adopts the self-developed RHAM technology, which enables the accuracy of test results comparable to qPCR. It has the features of Palm-Sized, Rapid Detection, Easy to Use, and qPCR Level Accurancy. In combination with the reagents, the results can be obtained within 10-35 minutes. It is an ideal device for field testing, primary care, hospital outpatient clinics, home testing scenarios, etc.