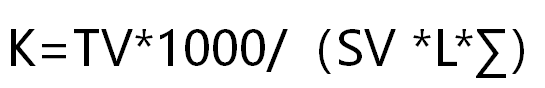Calculation of Biochemistry Analyzer K-value
When we measure a specimen, whether you use a manual method or a
fully automated biochemistry analyzer, the value determined is just an absorbance. This absorbance means nothing to us. We need to convert this absorbance into a concentration or enzyme activity. Then we have to multiply it by a K value. The calculated and printed result will be meaningful to us.
The K-value is what we find by calibrating. The general minimum requirement is to have a reagent blank and a standard.
The concentration of the standard is known. These two absorbances can be measured by a biochemical analyzer. This gives a K value. Whatever the specimen, multiplying its absorbance by the K value gives us the answer. Therefore, the K value is very decisive and can determine the accuracy of a specimen.
Determinants of Biochemistry Analyzer K-value
Let's look at what affects the K value.
First, the concentration of the standard solution must be accurate (the standard solution should preferably be serum-based as well as the specimen).
Second, the absorbance of the standard solution and the absorbance of the reagent blank must be accurate. The absorbance is affected by the condition of the biochemical machine, the condition of the reagents, and if your instrument is fairly stable, the reagents are a major factor affecting the K-value.
Defense of Biochemistry Analyzer K-value
Usually we use a QC serum to check. It is better to have two levels of QC to check. If the QC results are good, you can say that the K value is accurate and the patient's results calculated with this K value are accurate. So the K value is very critical.
The K value actually represents the slope and the intercept represents the reagent blank, which changes every day, so the stability of the K value determines your biochemistry analyzer and reagents. If the instrument and reagents are very stable, then the K value is also very stable.
How often is the calibration appropriate? It depends on the stability of your reagents. Some projects can be solved by doing a reagent blank for poor QC results, while others require two-point calibrations.
How to determine the K value for enzyme items?
One is the theoretical K value calculated.
The other is the K value derived from the calibration solution with the enzyme project.
At present, many companies can provide calibration solutions with multiple items, not only for substrate items, but also for enzyme items. After the calibration, with the calibration result, how to judge the good or bad result?
If you use the original reagent, most of the items in the original parameters will provide a range of factors. If the K value after calibration is exactly the median of the factor range, congratulations, it means that the state of your biochemistry analyzer is exactly the same as the state in the manufacturer's laboratory. The condition of the instrument, the condition of the reagents, the condition of the water machine, etc. are all very, very good.
If you use an instrument or reagent, the manufacturer does not provide a range of factors, what can you do?
The method is to figure it out yourself. Record the factor once for each calibration. Accumulate enough times and you will be able to determine the range of factors yourself. If the wrong calibration solution is used, the calibration will fail.
Older models of biochemistry analyzers still use the K value of the last successful calibration to calculate patient results. Newer models have no results. Therefore, it is important to observe the K value after calibration and whether the calibration was successful or not.