release time:2021-07-26 15:11:59
Common in vitro diagnostic devices:
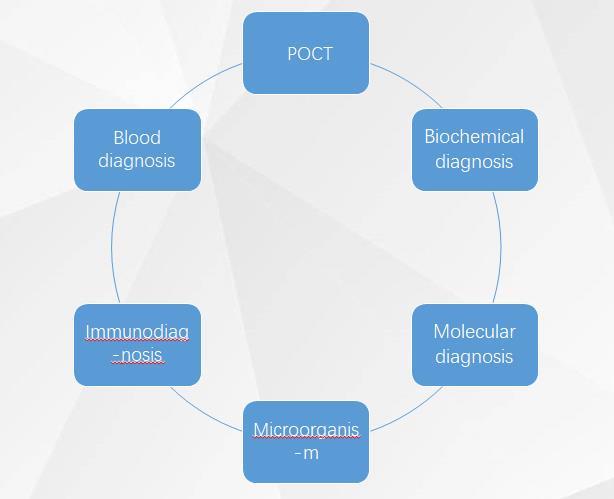
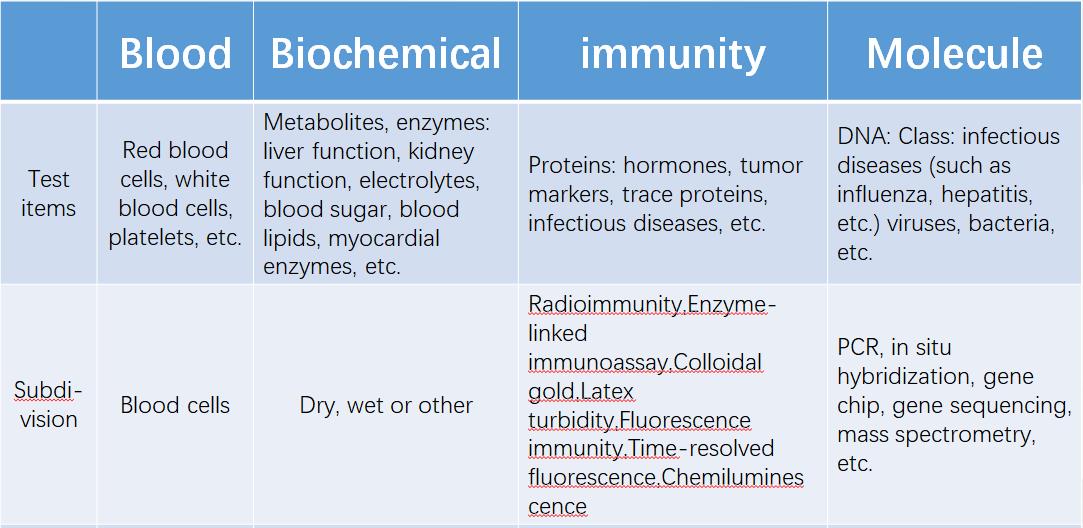
According to the detection category, it can be divided into blood test, biochemical diagnosis, immunodiagnosis, molecular diagnosis, microbiological diagnosis, POCT, etc.
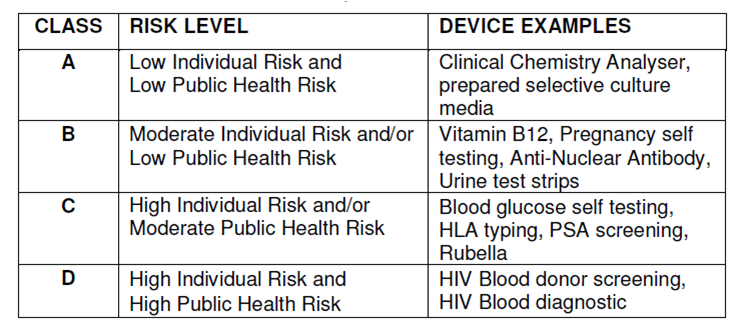
The FDA classifies medical devices (including IVD products) into Class I, Class II, or Class III based on the level of regulatory control required to reasonably ensure safety and effectiveness. The classification of IVD (or other medical devices) determines the appropriate pre-marketing process.

2022-08-03
A clinical chemistry analyzer is a machine used in the diagnosis and treatment of disease. It is used to measure various chemicals in the blood, urine, or other body fluids. This information can be used to help identify and diagnose problems. Clinical chemistry analyzers are an important part of modern healthcare. They allow for fast and accurate analysis of patient samples. This helps ensure that patients receive the best possible care.
2022-02-22
Some enzymes with relative molecular mass much larger than normal enzyme molecules can sometimes be found in the serum, which are usually called giant enzymes or macroenzymes for short.

2021-09-15
The popularity of fully automated biochemical analyzers has greatly improved the speed of hospital testing. However, improper operation makes the biochemical test results of the test instrument error. Also yes, the convenience of the biochemical analyzer is meaningless. Even lead doctors to make the wrong diagnosis.